About Us
Advancing Dermatological Clinical Research, Together
At Blueskin, we specialize in designing and delivering clinical trials that move dermatological research forward. We combine indication-specific insight with operational precision to support sponsors in developing better treatments, faster.
With a flexible model, dedicated team, and access to integrated trial services through Sanos, we ensure that every clinical study is built for efficiency, quality, and meaningful clinical outcomes.
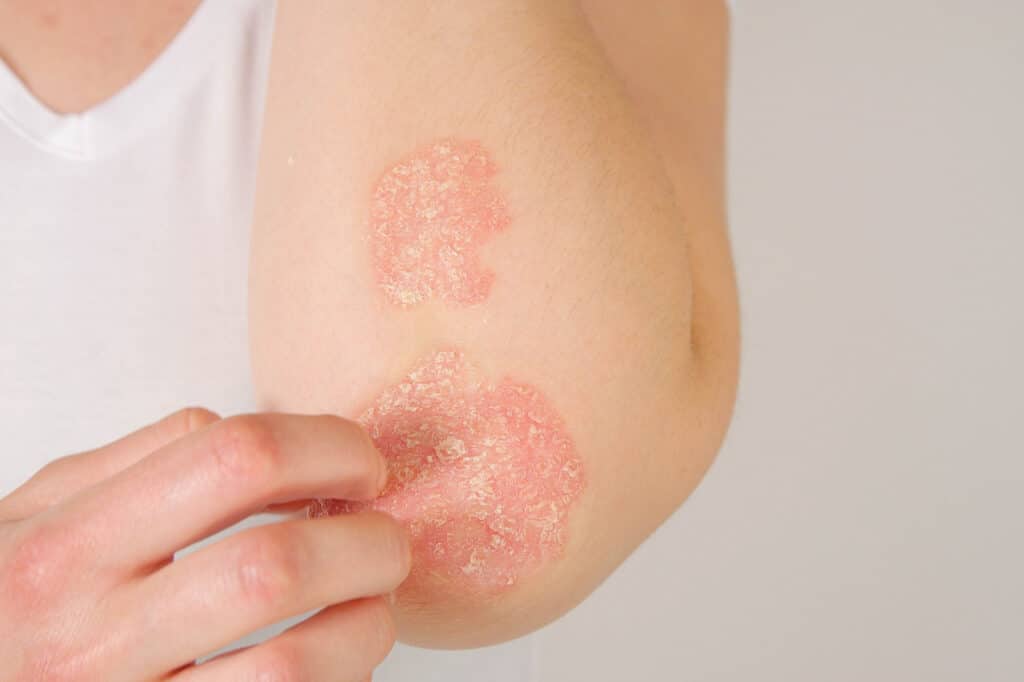
Expertise
A Niche Focus
At Blueskin, our work is driven by a true passion and curiosity for advancing dermatology. As a niche CRO fully dedicated to dermatological conditions, we bring indication-specific insight that helps shape smarter clinical trial design, refine clinical endpoints, and generate meaningful data.

Approach
Built Around You
At Blueskin, our approach is grounded in true partnership. We tailor each clinical trial with a flexible mindset, prioritizing sponsor objectives, while maintaining a patient-centric focus to ensure a smooth collaboration, add early value, and set the study up for long-term success.

Services
Seamless Trial Execution
Blueskin is a full-service CRO and integrates seamlessly with dedicated clinical research sites, clinical trial supply, patient recruitment, and eCOA through Sanos – delivering consistent, end-to-end support across every stage of your clinical trial.
What Drives Our Approach
At Blueskin, we build every clinical trial around what matters most: the science, the patients, and the outcomes.
We do so by following our core values:
Curiosity
We stay curious, asking questions and challenging assumptions to drive smarter clinical dermatology research.
Agility
We adapt quickly, shaping each clinical study around sponsor needs, patient realities, and evolving trial conditions.
Empathy
We design with the patient in mind – ensuring accessibility, clarity, and dignity throughout the clinical trial.
Diligence
We hold ourselves to the highest scientific and operational standards, with every detail grounded in precision.
Collaboration
We believe in being true partners – open, transparent, risk-sharing and invested in the success of the sponsor.
The People at Blueskin
At Blueskin, we combine dermatological specialization with a strong, supportive culture that puts both science and people first. Our team collaborates across clinical operations, trial design, regulatory and safety, and patient engagement – each person contributing to clinical trials that are built with care, clarity, and purpose.
For sponsors, this means a dedicated partner that’s responsive, invested, and ready to deliver. For our people, it’s a workplace where collaboration is real, ideas are valued, and everyone has the space to grow.


